Write a Balanced Equation for the Combustion of Propane
Think of the given problem. A C3H8 5O2 4H2O 3CO 2 b Combustion 3.
What Is The Balanced Chemical Equation For The Combustion Of Propane Quora
4 mol C3H8 x 5 mol O2 20 mol O2.
. Learn about and revise polluting the atmosphere with this BBC Bitesize GCSE Chemistry Eduqas study guide. Ethanol and oxygen are the reactants. Consider the equation for the combustion of acetone C3H8O the main ingredient in nail polish remover.
Iron reacts with oxygen gas to produce Iron III oxide. Write the balanced complete ionic equation for the reaction when copper I acetate and calcium sulfide are mixed in aqueous solution. Limiting reagent is the reactant which limits the progress of a chemical reaction.
Br-aq Ag aq -- AgBrs Write the balanced net ionic equation for the reaction when aqueous KBr and aqueous AgC2H3O2 are mixed in solution to form aqueous KC2H3O2 and solid AgBr. Get help with your Stoichiometry homework. 2H₂g O₂g 2H₂Og The mole ratio between O₂ and H₂O is 1 mol O₂2 mol H₂O.
C3H8O l O2 g -- CO2 g H2O g Hrxn -1790 kJ. For each of the following chemical reactions write a word equation a skeleton equation and a balanced chemical equation. A Write an equation to represent the standard enthalpy change of formation of propane.
Write the balanced equation for the process of combustion of ethanol. The formula for ethanol is ceC_2H_5OH. Ethanol is a fuel source in an alcohol lamp.
Write the balanced reaction for the combustion of propane C3H8. A Write a balanced thermochemical equation for the reaction. The given product is H2g and based on knowledge of redox.
Write a balanced equation for the complete combustion of methane CH 4 found in natural gas. Chemists often write this as. This balanced chemical equation summarizes the chemical reaction involved in burning methane.
For instance when you burn methane natural gas in your stove the methane is reacting with oxygen to form carbon dioxide and water. The mole ratio between H₂ and H₂O is 2 mol H₂2 mol H₂O. One type of LPG consists mainly of propane C3H8.
Write a balanced equation after determining the products and reactants. A Write and balance the given equation. CH 4 2 O 2 CO 2 2 H 2 O energy.
Ethanol can be used as a fuel source in an alcohol lamp. The formula for ethanol is given by C 2 H 5 OH. Be sure to show the state of each reactant and prod- uct.
C3H8 5 O2 3 CO2 4 H2O_____ How many moles of oxygen are necessary to react completely with 4 moles of propane. Write the balanced equation for the combustion of ethanol. In this situation since we assume copper does not react the reactants are only H aq and Fes.
Stoichiometry Questions and Answers. B Draw an enthalpy diagram for the reaction. B Indicate the type of chemical reaction represented.
Indicate the physical states of the reactants and products using the abbreviations s l g or aq for solids liquids gases or aqueous solutions respectively. When a fuel containing carbon combusts in the presence of excess oxygen it will produce carbon dioxide gas CO 2g. How many moles of O₂ are required to form 500 moles of H₂O.
Access the answers to hundreds of Stoichiometry questions that. This combustion occurs when something is ignited to produce heat light and sound energy as in firecrackers. If two atoms must combine at a 1 to 1 ratio but there is an unequal amount of 1 atom then the reaction will stop when the atom with less quantity runs out.
Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations. Write the balanced chemical equation for the neutralization reaction that occurs when an aqueous solution of hydrobromic acid HBr is mixed with an aqueous solution of lithium hydroxide LiOH. All the fuels both renewable and non-renewable that we have been discussing contain carbon.
Propane C 3H8 reacts with oxygen gas to produce carbon dioxide and water. Liquefied petroleum gas LPG is a type of fuel that can be used in heating appliances cooking equipment and vehicles. As with a hydrocarbon the products of the combustion of an alcohol are carbon dioxide and water.
Another way of expressing this is to say that there is a low fuel to oxygen ratio or fueloxygen is low. Nature of Products of Combustion of Fuels. For example in the reaction.
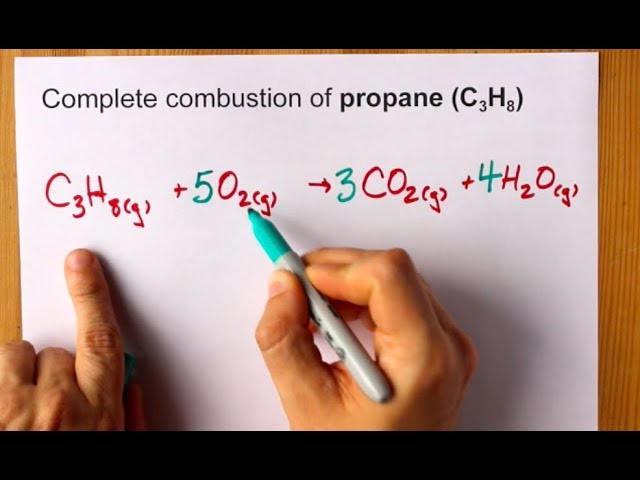
Here are the equations for the complete combustion of propane. B Write an equation to represent the standard enthalpy change of combustion of propane. A 4Fe 3O2 2Fe 2O3 b Synthesis 2.
If you need more help writing formulas or determining the state of a substance refer to Chapters 7 and 8 and the periodic table on pages 178179.
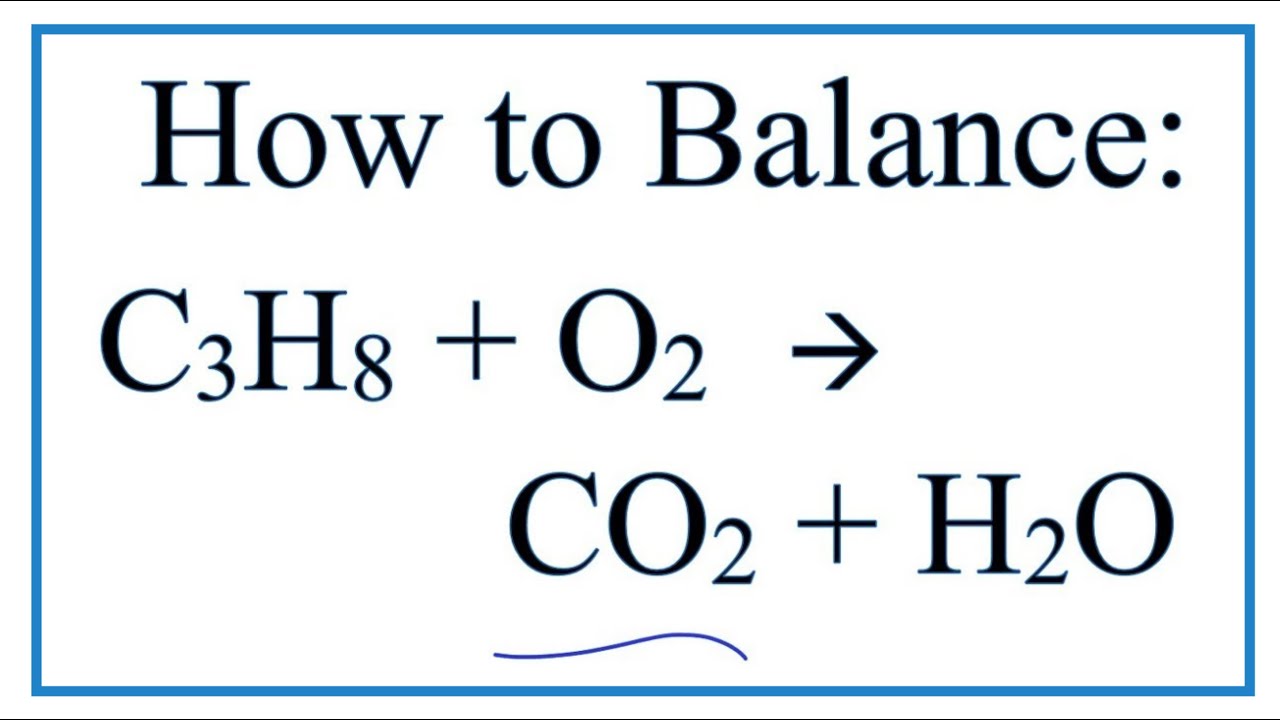
How To Balance C3h8 O2 Co2 H2o Propane Combustion Reaction Youtube

Complete Combustion Of Propane C3h8 Balanced Equation Youtube
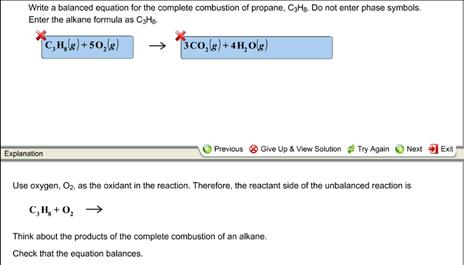
Solved Write A Balanced Equation For The Complete Combustion Of Propane 1 Answer Transtutors
Comments
Post a Comment